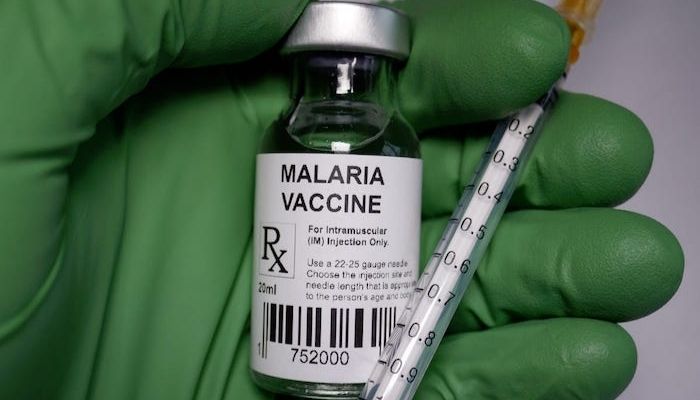
The National Agency for Food and Drug Administration and Control has approved the R21 malaria vaccine manufactured by the Serum Institute of India. This makes Nigeria the second country, after Ghana, to approve the new malaria vaccine developed at the University of Oxford, .
The Director General of NAFDAC, Prof Mojisola Adeyeye, speaking at a press briefing in Abuja, says the vaccine is indicated for the prevention of clinical malaria in children from 5 months to 36 months of age.
She says Nigeria expects to get at least 100,000 doses of the vaccine in donations soon before the market authorisation will start making other arrangements with the National Primary Health Care Development Agency.
READ ALSO: FG To Address Aviation Demands Soon, Calls For Strike Cancellation
Professor Adeyeye, while speaking to the press, added that the dossier of the vaccine was subjected to independent review at many levels, namely NAFDAC’s Vaccine Advisory Committee, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human use guidelines, the European Medicines Agency guidelines, scientific rigour on the vaccine, and best research and manufacturing governance.
She says NAFDAC in exercising its mandate as stipulated by its enabling law, NAFDAC Act CapN1, LFN 2004 is granting registration approval for R21 Malaria Vaccine (Recombinant, Adjuvanted) manufactured by Serum Institute of India Pvt. Ltd.





Leave feedback about this
You must be logged in to post a comment.